Companion Diagnostics Market Report 2025 | Growth, Trends & Forecast by 2033
Market Overview:
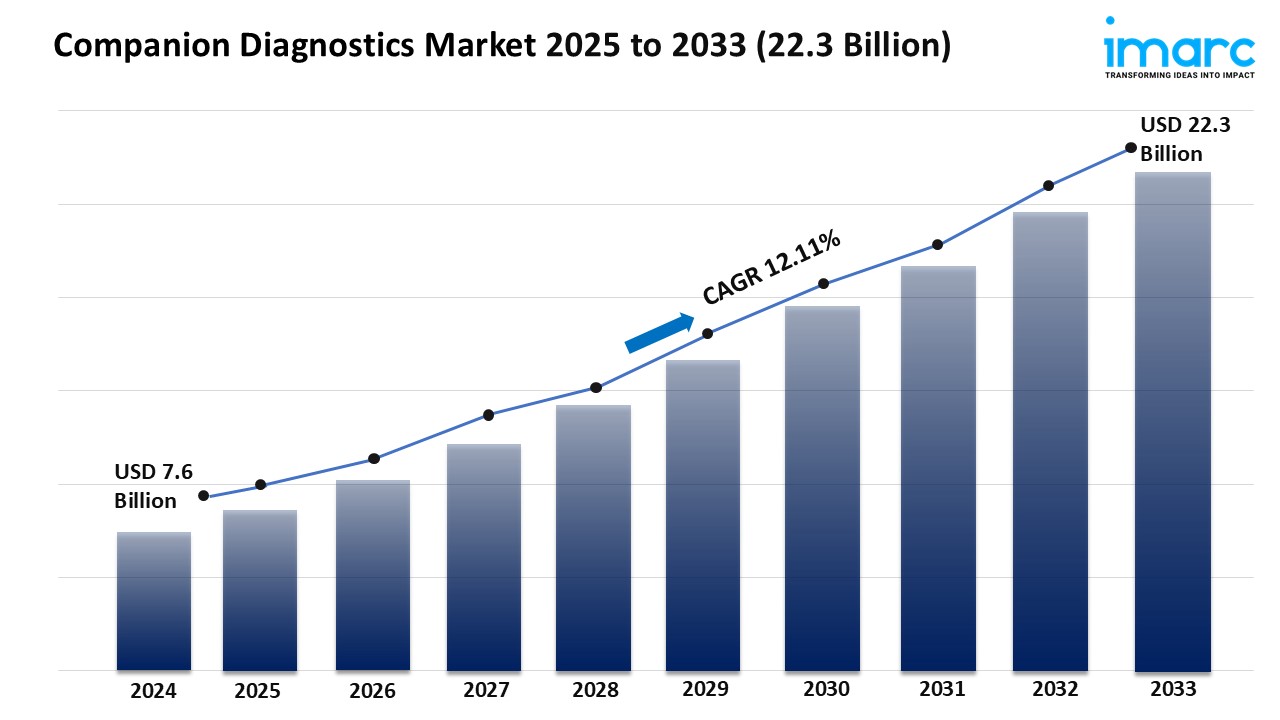
The companion diagnostics market is experiencing rapid growth, driven by precision medicine expansion, oncology dominates demand, and regulatory and reimbursement support. According to IMARC Group’s latest research publication, “Companion Diagnostics Market Report by Product & Service (Assays, Kits and Reagents, Software and Services), Technology (Immunohistochemistry (IHC), Polymerase Chain Reaction (PCR), In-situ Hybridization (ISH), Real-time PCR (RT-PCR), Gene Sequencing, and Others), Indication (Cancer, Neurological Diseases, Infectious Diseases, Cardiovascular Diseases, and Others), End User (Pharmaceutical & Biopharmaceutical Companies, Reference Laboratories, Contract Research Organizations, and Others), and Region 2025-2033.” The global companion diagnostics market size reached USD 7.6 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 22.3 Billion by 2033, exhibiting a growth rate (CAGR) of 12.11% during 2025-2033.
This detailed analysis primarily encompasses industry size, business trends, market share, key growth factors, and regional forecasts. The report offers a comprehensive overview and integrates research findings, market assessments, and data from different sources. It also includes pivotal market dynamics like drivers and challenges, while also highlighting growth opportunities, financial insights, technological improvements, emerging trends, and innovations. Besides this, the report provides regional market evaluation, along with a competitive landscape analysis.
Grab a sample PDF of this report: https://www.imarcgroup.com/companion-diagnostics-market/requestsample
Our report includes:
- Market Dynamics
- Market Trends and Market Outlook
- Competitive Analysis
- Industry Segmentation
- Strategic Recommendations
Factors Affecting the Growth of the Companion Diagnostics Industry:
- Precision Medicine Expansion
The increasing adoption of precision medicine processes is catalyzing the growth of companion diagnostics as they provide treatment options to the specific genetic or molecular profile of a patient’s condition. Pharmaceutical companies to a growing extent are embedding companion diagnostics relating to drug development processes so that drug manufacturers can identify responders to their drug in order to optimize therapeutic effect and minimize side effects. Regulatory bodies are also providing pathways for the adoption of these diagnostics as they’ve provided expedited approval rates for targeted therapies. This pattern has begun to develop in situations and outset of oncology-based treatments where directed based molecular treatments are shifting the practice of cancer care. As precision medicine continues to be adopted more widely, companion diagnostic processes will likely trend toward being a compulsory aspect of prescribing because providers can tailer treatment more effectively and safely with minimal trial and error.
- Oncology Dominates Demand
Oncology is still the largest focus area for companion diagnostic applications, driven primarily by the increase of targeted cancer therapies. Tests that determine the presence or absence of biomarkers such as HER2, EGFR, and PD-L1 are important for identifying eligible patients and measuring treatment response to therapies relying on these biomarkers. The growing incidence of cancer and availability of genomic studies will only add to the accelerating growth of companion diagnostics in oncology. Drug developers have made companion diagnostics a top priority area, as the next generation of immunotherapies, along with the emergence of small-molecule inhibitors, continue to grow. With cancer care continuing to move toward more precision treatment, the need for companion diagnostics will continue to grow as they cement their place as the standard of care in oncology.
- Regulatory and Reimbursement Support
Favorable regulatory policies and better reimbursement frameworks have driven the adoption of companion diagnostics. The FDA and EMA now specifically require or recommend companion diagnostics in certain targeted therapies, allowing for safer and properly targeted treatments. Payers are also expanding their coverage because they recognize that companion diagnostics are valuable in lowering unnecessary drug costs. In addition, an increasing number of biomarker-based tests are being approved through expedited review paths, promoting new growth. With a continued transition to value-based systems of care, companion diagnostics are increasingly being seen as cost-effective diagnostic tools. This is opening the door to their introduction into more clinical environments and even further growth on the market.
The companion diagnostics market report provides a comprehensive overview of the industry. This analysis is essential for stakeholders aiming to navigate the complexities of the biochar market and capitalize on emerging opportunities.
Leading Companies Operating in the Companion Diagnostics Industry:

- Abbott Laboratories
- Agilent Technologies
- BioMerieux
- Danaher Corporation
- Roche Holding AG
- Myriad Genetics Inc.
- Siemens Healthcare
- Thermo Fisher Scientific Inc.
Companion Diagnostics Market Report Segmentation:
By Product & Service:

- Assays, Kits and Reagents
- Software and Services
Assays, kits, and reagents dominate the market due to their critical role in biomarker detection and enabling targeted therapies.
By Technology:
- Immunohistochemistry (IHC)
- Polymerase Chain Reaction (PCR)
- In-situ Hybridization (ISH)
- Real-time PCR (RT-PCR)
- Gene Sequencing
- Others
Polymerase chain reaction (PCR) leads the segment owing to its widespread use in mutation detection and easy availability of diagnostic kits.
By Indication:
- Cancer
- Lung Cancer
- Breast Cancer
- Colorectal Cancer
- Gastric Cancer
- Melanoma
- Others
- Neurological Diseases
- Infectious Diseases
- Cardiovascular Diseases
- Others
Cancer holds the largest share, driven by high disease prevalence and increasing approvals of companion diagnostics for oncology treatments.
By End User:
- Pharmaceutical & Biopharmaceutical Companies
- Reference Laboratories
- Contract Research Organizations
- Others
Pharmaceutical & biopharmaceutical companies account for the highest demand as they integrate companion diagnostics into drug development.
Regional Insights:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America leads the market, supported by advanced healthcare infrastructure, strong regulatory support, and high adoption of precision medicine.
Research Methodology:
The report employs a comprehensive research methodology, combining primary and secondary data sources to validate findings. It includes market assessments, surveys, expert opinions, and data triangulation techniques to ensure accuracy and reliability.
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145
Related Article: Debt Collection Software Market Growth, Share, and Trends Forecast 2025-2033