Streamlining Recombinant Protein Production for Therapeutic Use
In the rapidly evolving field of biotechnology, the demand for high-quality recombinant proteins is more critical than ever, particularly for therapeutic applications. Protein production services play a vital role in the development of these proteins, which are essential for research, diagnostics, and therapeutics. This blog explores how to streamline recombinant protein production, ensuring efficiency and reliability while maintaining high-quality standards.
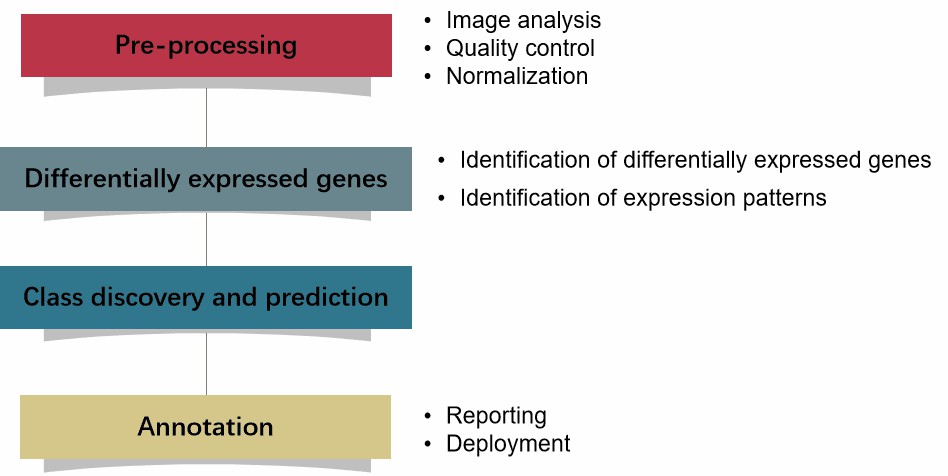
Understanding Recombinant Protein Production
Recombinant protein production involves the use of genetically engineered organisms to produce proteins that may not be naturally abundant or that require modification for therapeutic use. Common expression systems include bacteria, yeast, insect cells, and mammalian cells. Each system offers distinct advantages and challenges, making it essential to choose the right one for specific applications.
By optimizing the selection and cultivation of host cells, as well as the conditions under which proteins are produced, scientists can enhance yield and functionality. Therefore, leveraging professional lab research in protein production services is crucial for achieving the desired outcomes in therapeutic applications.
Key Steps in Streamlining Protein Production Services
1. Selecting the Right Expression System
The first step in recombinant protein production is selecting the appropriate expression system. Factors to consider include:
- Protein Complexity: Some proteins require post-translational modifications, which are more efficiently performed in mammalian cells.
- Cost and Time Efficiency: Bacterial systems like E. coli can be cost-effective for producing simpler proteins.
- Yield Requirements: Certain systems may offer higher yields depending on the nature of the target protein.
For high-throughput needs, consider protein production services that offer a range of expression systems tailored to specific protein characteristics. For more information, check over here to learn about various systems and their applications.
2. Optimizing Cloning and Expression
Once the expression system is selected, the next step is to clone the gene of interest into an appropriate vector. The following strategies can enhance this process:
- Codon Optimization: Modifying the DNA sequence to match the host organism’s preferred codon usage can significantly improve protein yield.
- Vector Selection: Choose vectors that facilitate high expression levels and include tags for easier purification.
- Induction Conditions: Carefully optimize induction parameters, such as temperature, duration, and inducer concentration, to maximize protein output.
Utilizing protein production services can provide expertise in vector design and optimization techniques, elevating your research to new heights. Click this link here now for more detailed strategies on cloning and expression optimization.
3. Enhancing Cell Culture Conditions
The growth conditions of the host cells can dramatically affect the quality and yield of the recombinant protein. Consider the following aspects:
- Media Selection: Use rich media with appropriate supplements to enhance cell growth and protein production.
- pH and Temperature Control: Maintain optimal pH and temperature for the specific cell type, as deviations can stress the cells and reduce protein yields.
- Fed-Batch Cultures: Implement fed-batch strategies to continuously supply nutrients, supporting prolonged cell growth and higher protein yields.
Professional lab research has shown that these adjustments can lead to significant improvements in the production of high-quality recombinant proteins. For more information on optimizing cell culture conditions, click to read more.
4. Streamlining Purification Processes
Once proteins are produced, they require purification to remove host cell contaminants and achieve the desired level of purity. Streamlining purification processes involves:
- Affinity Chromatography: Utilize tags (e.g., His-tag, GST-tag) that allow for straightforward initial purification steps.
- Multi-Step Purification: Implement sequential chromatography techniques to refine the protein further, ensuring it meets therapeutic standards.
- Process Automation: Employ automated systems to increase throughput and reduce human error in purification protocols.
Outsourcing purification to protein production services can save time and resources, allowing researchers to focus on downstream applications. Get more information about the benefits of using specialized services for protein purification.
5. Implementing Quality Control Measures
Quality control is paramount in ensuring that recombinant proteins meet the stringent standards required for therapeutic use. Key quality control measures include:
- Characterization: Use techniques such as SDS-PAGE, Western blotting, and mass spectrometry to confirm protein identity and purity.
- Functional Assays: Conduct functional assays to verify that the produced proteins exhibit the expected biological activity.
- Stability Studies: Assess the stability of the protein under various conditions to ensure its viability during storage and transport.
Incorporating robust quality control measures into your protein production services will help ensure compliance with regulatory standards, thereby enhancing the trustworthiness of your therapeutic products.
6. Staying Compliant with Regulatory Standards
As recombinant proteins move toward therapeutic applications, compliance with regulatory standards becomes critical. Familiarize yourself with guidelines from organizations like the FDA and EMA regarding production practices. Implementing good manufacturing practices (GMP) in protein production is essential for ensuring safety and efficacy.
Protein production services that are familiar with these regulations can facilitate the transition from research to clinical use, streamlining the path to market for new therapeutic proteins.
Conclusion
Streamlining recombinant protein production is vital for accelerating the development of therapeutics that can address unmet medical needs. By selecting the right expression system, optimizing cloning and expression, enhancing cell culture conditions, streamlining purification processes, implementing quality control measures, and ensuring regulatory compliance, researchers can significantly improve the efficiency and quality of protein production.
Engaging with professional protein production services can provide the expertise and resources necessary to navigate this complex landscape successfully. Elevate your research and streamline your protein production processes by exploring the available services that cater to your specific needs. Go right here to learn more about how protein production services can transform your research into impactful therapeutic solutions. For any additional inquiries or guidance on recombinant protein production, please feel free to reach out to us!











Post Comment